Antimicrobial Peptides and Drug Discovery
In recent decades, the growing number of antibiotic-resistant pathogens has driven the need to develop agents with a unique mode of action. Antimicrobial Peptides (AMPs), also known as host defense peptides (HDPs), are an important model for the design of novel antibiotics due to their broad-spectrum antimicrobial potency. Because of this, the number of isolated AMPs/HDPs has increased considerably in recent years. Indeed, the AMP Database, curated by Prof. Guangshun Wang, contains more than 3700 entries.
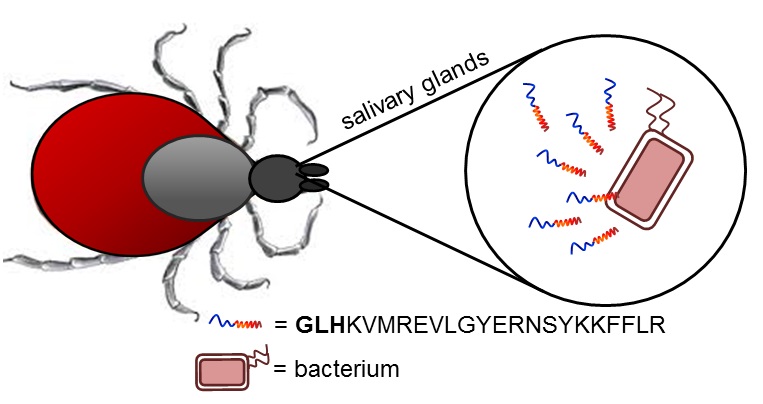
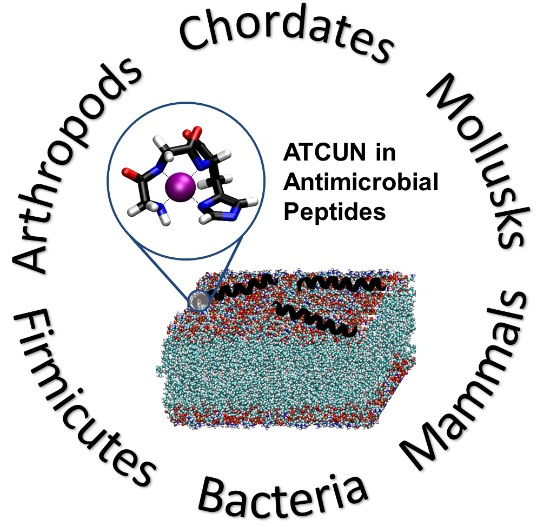
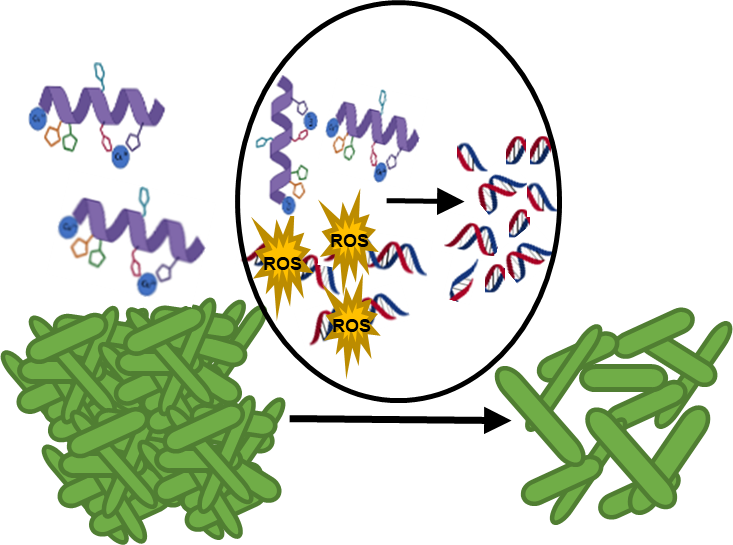
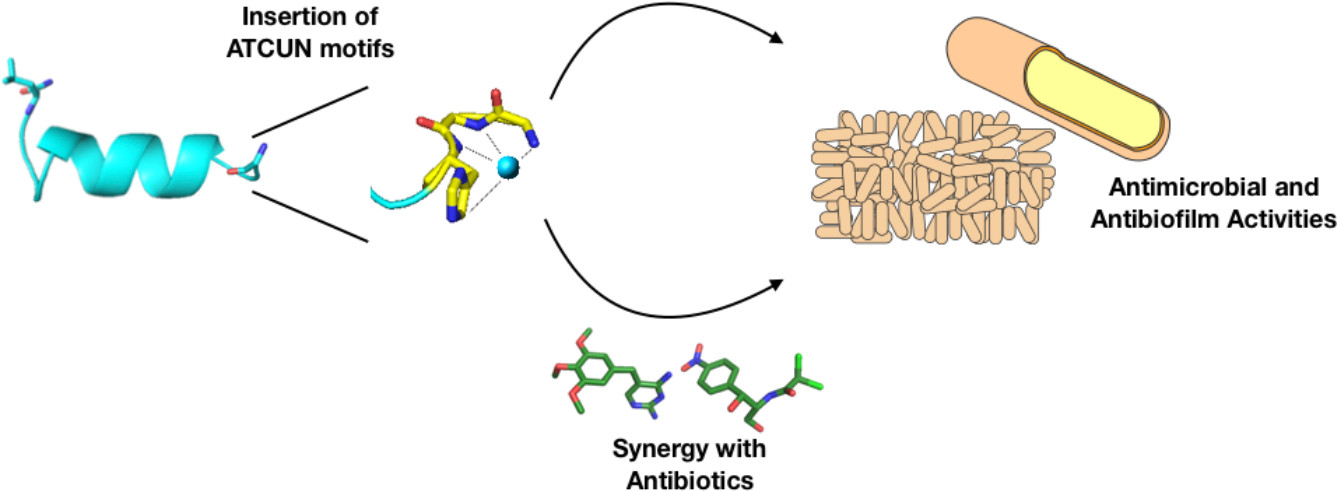
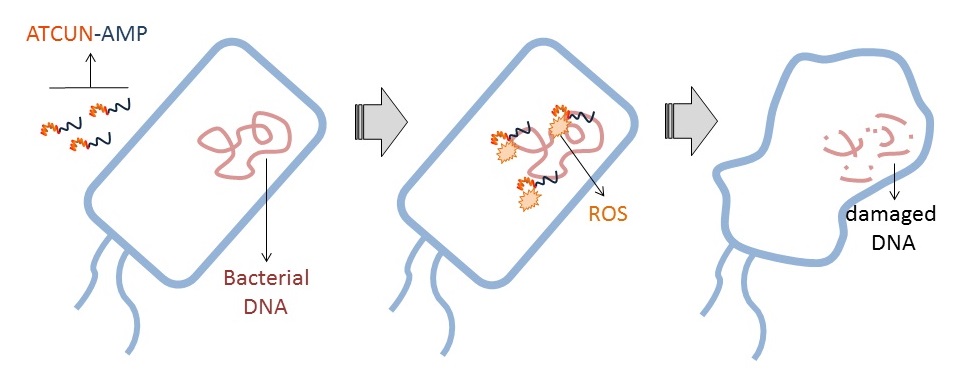
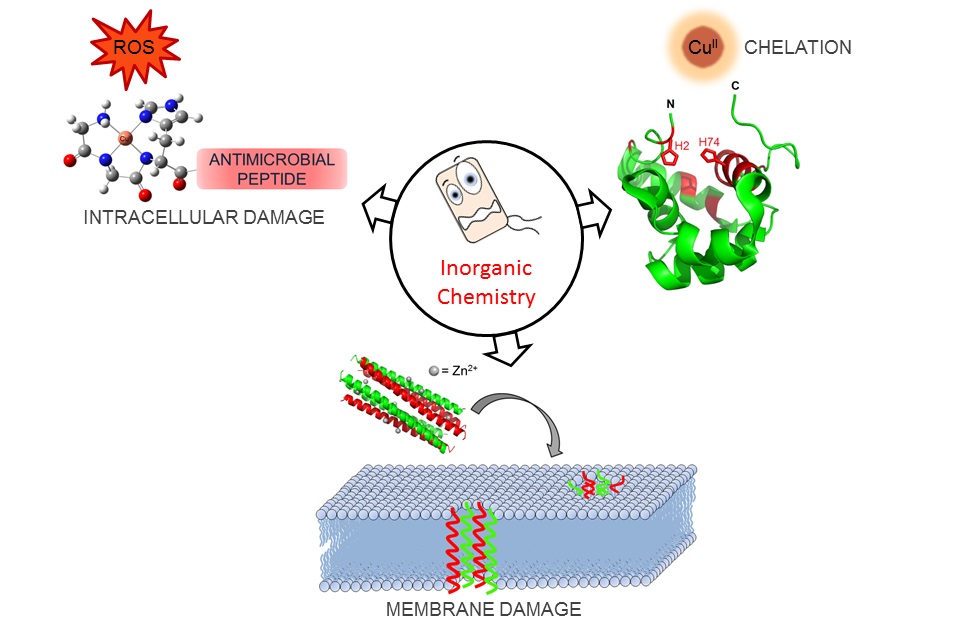
Our group’s goal is to unravel the chemistry of AMPs that use metal ions as co-factors to develop new antimicrobial agents. We were initially interested in AMPs that contain an Amino Terminal Copper and Nickel (ATCUN) motif. The ATCUN motif, H2N-AA1-AA2-His, is an structural feature present in proteins and has the ability to bind copper and nickel ions with high affinity. A perusal of the AMP literature and the AMP database indicates that many naturally-occurring AMPs contain an ATCUN motif (for a list of naturally occurring ATCUN-containing AMPs click here or check out our latest review in Chem. Rev. 2021). For example, ixosin (GLHKVMREVLGYERNSYKKFFLR), an AMP from the tick Ixodes sinensis contains the GLH ATCUN motif. We have recently demonstrated that ixosin does not only require the ATCUN motif to potentiate its own bactericidal activity, but the motif is key for its synergistic effect with a second salivary antimicrobial peptide (“Central Role of the Copper-binding Motif in the Complex Mechanism of Action of Ixosin: Enhancing Oxidative Damage and Promoting Synergy with Ixosin B”).
Figure 1. The ATCUN-containing AMP Ixosin is found in the salivary glands of the tick Ixodes sinensis.
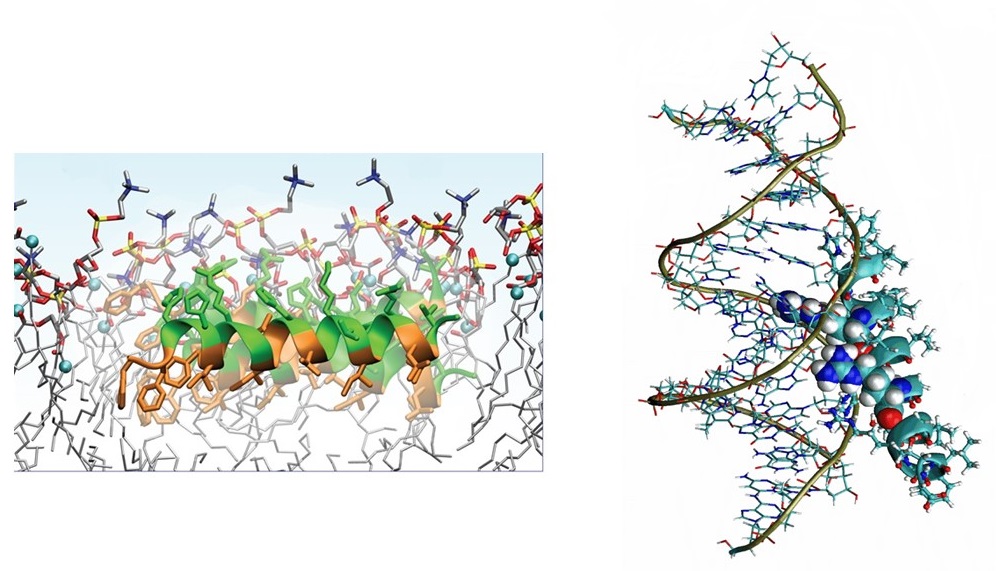
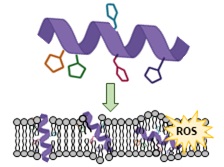
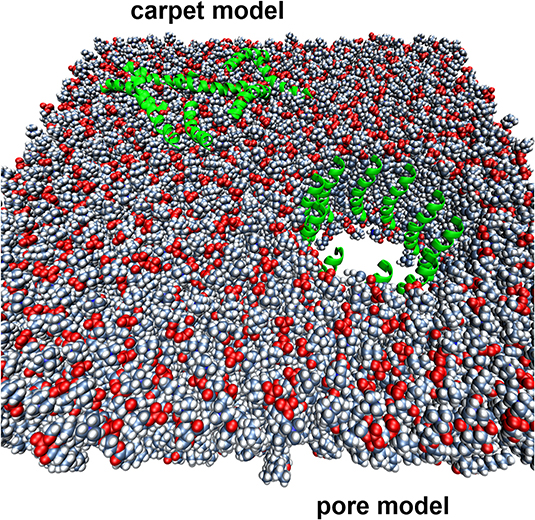
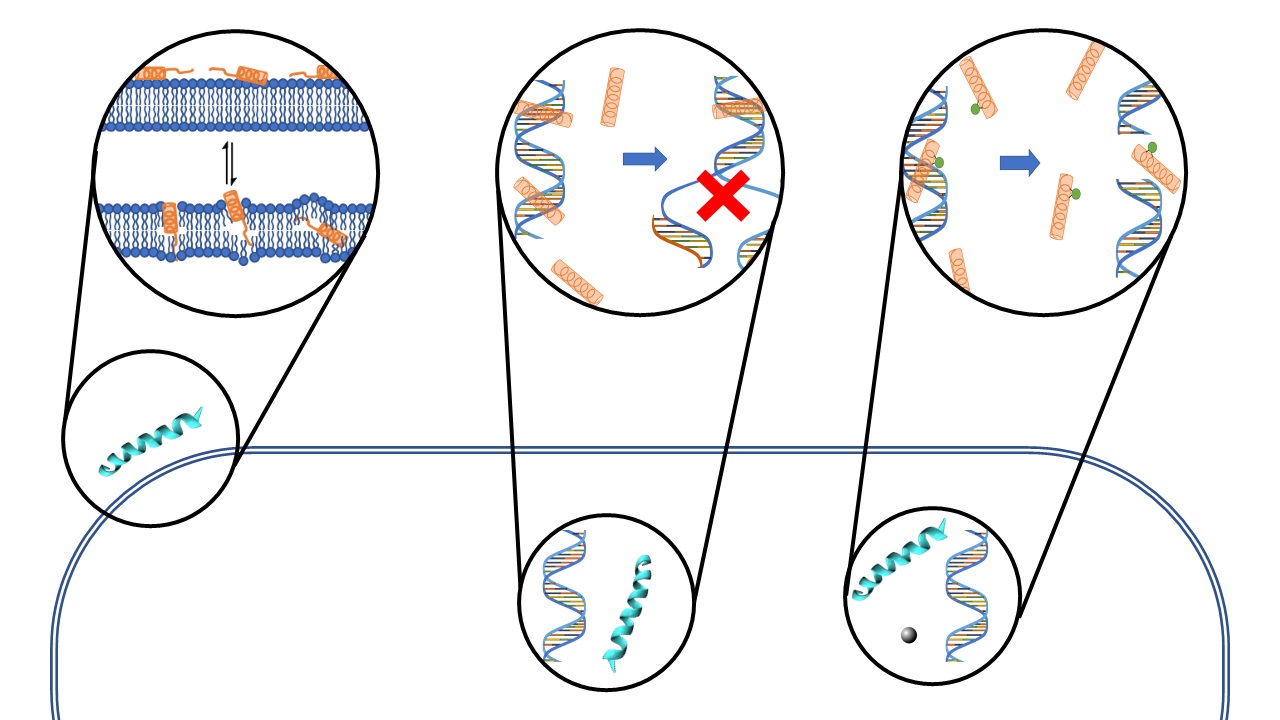
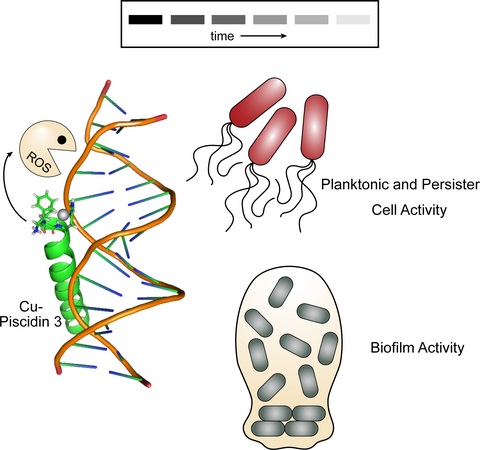
We are also studying a family of AMPs isolated from fish, known as piscidin 1 and piscidin 3. This work is performed in collaboration with Prof. Myriam Cotten (William & Mary), an expert on piscidin chemistry and biophysics. Our studies have revealed an interesting behavior between piscidin 1 and piscidin 3 as they are able to complement each other’s antimicrobial activities (“Complementary Effects of Host Defense Peptides Piscidin 1and Piscidin 3 on DNA and Lipid Membranes: Biophysical Insights into Contrasting Biological Activities”). Interestingly, whereas piscidin 1 prefers to bind and oxidize the bacterial membrane, piscidin 3
Figure 2. Computer-generated renditions of piscidin 1 binding to a model membrane (left, from Prof. Cotten’s publication: J. Am. Chem. Soc., 2014, 136 (9), pp 3491–3504) and to DNA (right, generated by Ruth Nussinov and Buyong Ma in FEBS 2017, 284, 3662-3683)
Animation of the use of metal ions by host defense peptides. Video created by Marcela Vertefeuille (illustration), Gillian Partyka (animation), and Cassidy Keller (animation). Created in the Scientific Visualization course led by Prof. Anna Lindemann. Based on the research performed in collaboration with Prof. Cotten.
Our publications on this field:
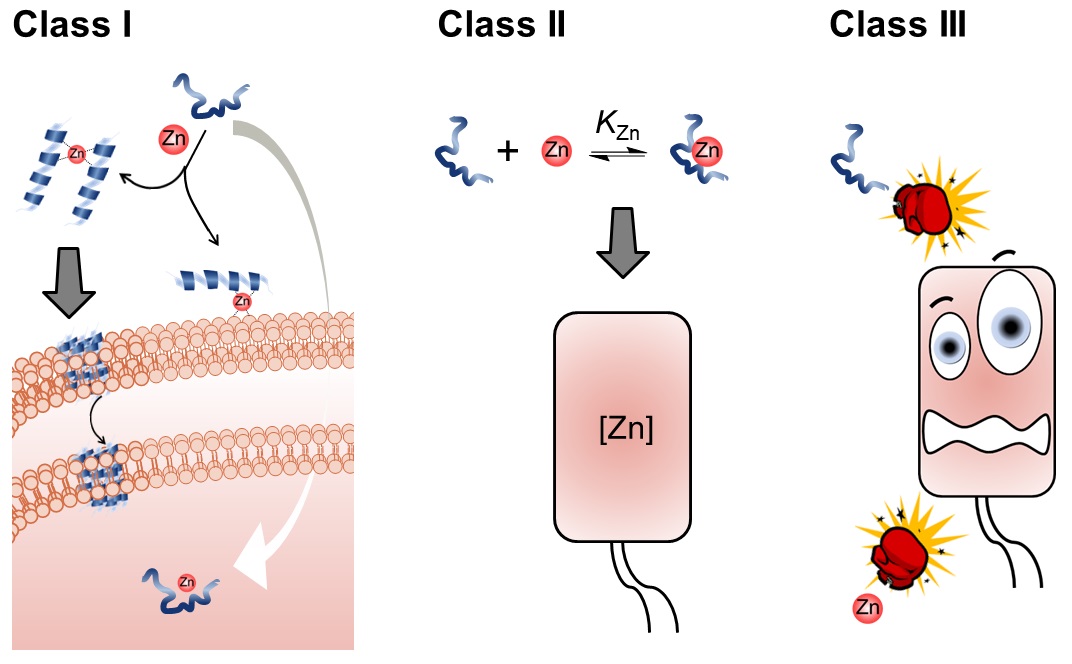
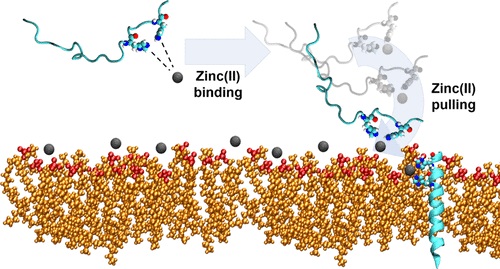
60. “The Synergy Between Zinc and Antimicrobial Peptides: An Insight into Unique Bioinorganic Interactions” Donaghy, C.;#, Javellana, J. G.;# Hong, Y.-J.; Djoko, K.;* Angeles-Boza, A. M.* Molecules 2023, 28(5), 2156.
58. “Metallated Anticancer Peptides: An Expanded Mechanism that Encompasses Physical and Chemical Bilayer Disruption“ Comert, F.; Heinrich, F.; Chowdhury, A.; Schoeneck, M.; Darling, C.; Anderson, K. W.; Libardo, M. D. J.; Angeles-Boza, A. M.; Silin, V.; Cotten, M. L.*; Mihailescu, M.* Sci. Reports 2021, 11, Article number: 12620.
57. “Antimicrobial susceptibility testing of antimicrobial peptides requires new and standardized testing structures” Meurer, M.; O’Neil, D. A.; Lovie, E.; Simpson, L.; Torres, M. D. T.; de la Fuente-Nunez, C.; Angeles-Boza, A. M.; Kleinsorgen, C.; Mercer, D. K.; von Koeckritz-Blickwede, M.* ACS Infect. Dis. 2021, 7, 2205–2208.
55. “Unraveling the implications of multiple histidine residues in the potent antimicrobial peptide Gaduscidin-1“ Portelinha, J.; Heilemann, K.; Jin, J.; Angeles-Boza, A. M. J. Inorg. Biochem. 2021, DOI: 10.1016/j.jinorgbio.2021.111391.
53. “Antimicrobial Peptides and Copper(II) Ions: Novel Therapeutic Opportunities“ Portelinha, J.†; Duay, S. S.†; Yu, S.; Heilemann, K.; Libardo, M. D. J.; Juliano, S.; Klassen, J. L.*; Angeles-Boza, A. M.* Chem. Rev. 2021, DOI: 10.1021/acs.chemrev.0c00921.
52. “Nuclease activity of the peptide Gad-1 clears Pseudomonas aeruginosa biofilms under cystic fibrosis conditions” Portelinha, J.; Angeles-Boza, A. M.* ChemBioChem 2021, DOI: 10.1002/cbic.202000816.
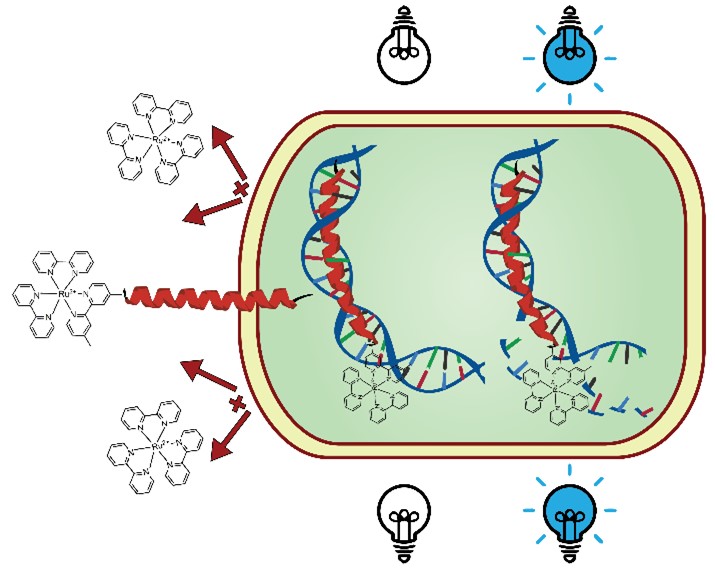
50. “Peptide-Ruthenium Conjugate as an Efficient Photosensitizer for the Inactivation of Multidrug Resistant Bacteria” Pierce, S.; Jennings, M.; Juliano, S.; Angeles-Boza, A. M. Inorg. Chem. 2020, 59 (20), 14866-14870.
49. “Antimicrobial Susceptibility Testing of Antimicrobial Peptides to Better Predict Efficacy” Mercer, D. K.*; Der Torossian Torres, M.; Duay, S. S.; Lovie, E.; Simpson, L.; von Köckritz-Blickwede, M.; de la Fuente-Nunez, C.; O’Neil, D. A.; Angeles-Boza, A. M. Frontiers in Cellular and Infection Microbiology 2020, DOI: 10.3389/fcimb.2020.00326
48. “A Potent Host Defense Peptide Triggers DNA Damage and is Active against Multi-Drug Resistant Gram-Negative Pathogens” Juliano, S. A.; Serafim, L. F.; Duay, S. S.; Heredia Chavez, M.; Sharma, G.; Rooney, M.; Comert, F.; Pierce, Scott; Radulescu, A.; Cotten, M. L.; Mihailescu, M.; May, E. R.; Greenwood, A. I.; Prabhakar, R.*; Angeles-Boza, A. M.* ACS Infect. Dis. 2020, 6, 1250–1263.
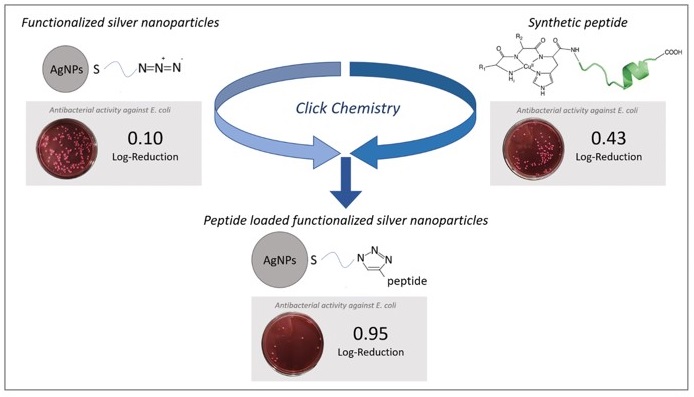
47. “Enhanced antimicrobial activity of silver nanoparticles conjugated with synthetic peptide by click chemistry” Gakiya-Teruya, M.; Palomino-Marcelo, L.; Pierce, S.; Angeles-Boza, A. M.; Vijay Krishna, V.; Rodriguez-Reyes, J. C. F.* J. Nanoparticle Res. 2020, 22, Article number: 90.
46. “Antimicrobial and antibiofilm activities of helical antimicrobial peptide sequences incorporating metal-binding motifs” Agbale, C.; Sarfo, J. K.; Galyuon, I. K. ; Juliano, S.; Silva, G. G. O; Buccini, D. F. ; Cardoso, M.; Torres, M.; Angeles-Boza, A.; de la Fuente-Nunez, C.; Franco, O. Biochemistry 2019, 58 (36), 3802-3812.
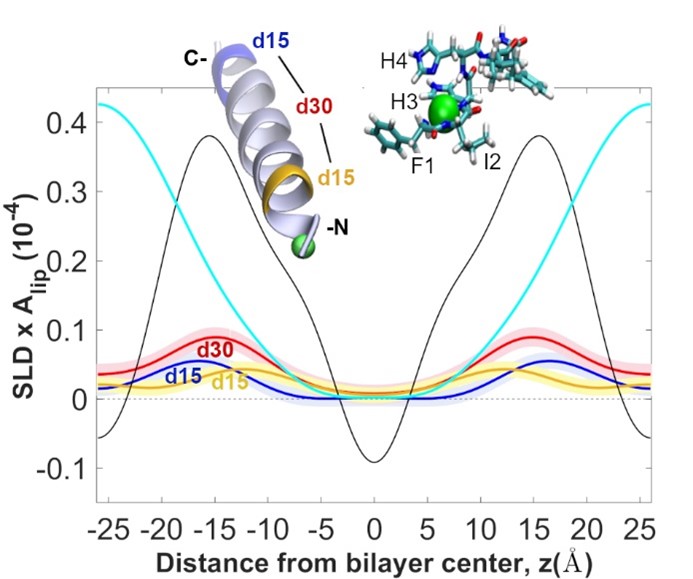
45. “Molecular Dynamics Investigation into the Effect of Zinc(II) on the Structure and Membrane Interactions of the Antimicrobial Peptide Clavanin A” Duay, S.; Sharma, G.; Prabhakar, R.; Angeles-Boza, A. M.*; May, E. R.* J. Phys. Chem. B 2019, 123(15), 3163–3176.
42. “Phagosomal Copper–promoted Oxidative Attack on Intracellular Mycobacterium tuberculosis” M. Libardo, D. J.; Fuente-Nunez, C.; Anand, K.; Krishnamoorthy, G.; Kaiser, P.; Pringle, S. C.; Dietz, C.; Pierce, S.; Smith, M. B.; Barczak, A. K.; Kaufmann, S. H. E.; Singh, A.; Angeles-Boza, A. M. ACS Infect. Dis. 2018, 4 (11), 1623–1634.
Press release: A Copper Bullet for Tuberculosis
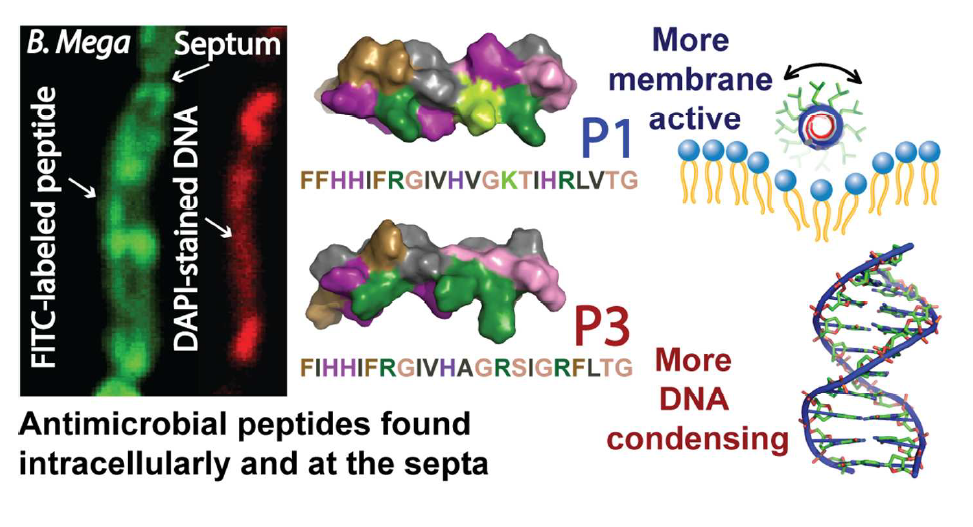
40. “Nuclease Activity Gives an Edge to Host-Defense Peptide Piscidin 3 over Piscidin 1, Rendering it more Effective against Persisters and Biofilms” Libardo, M. J.; Bahar, A. A.; Ma, B.; Fu, R.; Zhao, J.; Nussinov, R.; Ren, D.; Angeles-Boza, A. M.*; Cotten, M. L.* FEBS J. 2017, 284, 3662-3683
Highlighted in: http://www.aquahoy.com, https://phys.org/news
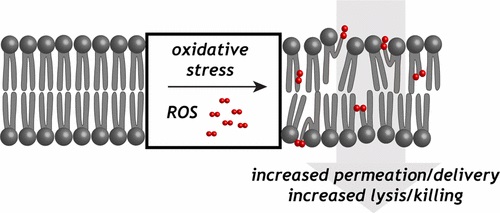
37. “How Does Membrane Oxidation Affect Cell Delivery and Cell Killing?“Libardo, M. D. J.; Wang, T.-Y.; Pellois, J. P.*; Angeles-Boza, A. M.* Trends Biotech. 2017, 35, 686-690.
36. “Cytotoxic property of Streptocaulon baumii extracts and their isolated compounds against different human cancer cell lines.pdf” Bartolome, Arlene P.; Villasenor, Irene M.; Lin, Yea-Lih; Libardo, M. Daben J.; Angeles-Boza, A. M.; Yang, Wen-Chin Phil. Sci. Lett. 2017, 10, 89-97.
35. “Membrane oxidation in cell delivery and cell killing applications” Wang, T.-Y.; Libardo, M. D. J.; Angeles-Boza, A. M.*; Pellois, J. P.* ACS Chem. Biol. 2017, 12, 1170-1182.
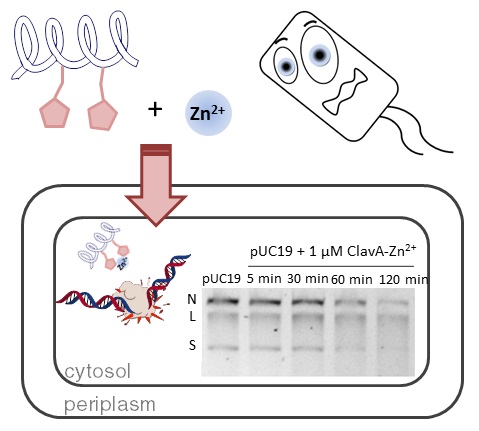
33. “Exploration of the Innate Immune System of Styela clava: Zn2+ Binding Enhances the Antimicrobial Activity of the Tunicate Peptide Clavanin A” Juliano, S. A.; Pierce, S.; deMayo, J. A.; Balunas, M. J.; Angeles-Boza, A. M.* Biochemistry 2017, 56 (10), 1403–1414. (Click here if your school does not have access to Biochemistry).
27. “Central Role of the Copper-binding Motif in the Complex Mechanism of Action of Ixosin: Enhancing Oxidative Damage and Promoting Synergy with Ixosin B” Libardo, M. D. J.; Gorbatyuk, V. Y.; Angeles-Boza, A. M. ACS Infectious Diseases 2016, 2(1), 71-81.
26. “Complementary Effects of Host Defense Peptides Piscidin 1and Piscidin 3 on DNA and Lipid Membranes: Biophysical Insights into Contrasting Biological Activities” Hayden, R. M.; Goldberg, G. K.; Ferguson, B. M.; Schoeneck, M. W.; Libardo, M. D. J.; Mayeux, S. E; Shrestha, A.; Bogardus, K. A.; Hammer, J.; Pryshchep, S.; Lehman, H. K.; McCormick, M. L; Blazyk, J.; Angeles-Boza, A. M.; Fu, R.; Cotten, M. L.* J. Phys. Chem. B 2015, 119(49), 15235-15246.
25. “Hybrid peptide ATCUN-sh-Buforin: Influence of the ATCUN charge and stereochemistry on antimicrobial activity” Libardo, M. D. J.; Paul, T. J.; Prabhakar, R.; Angeles-Boza, A. M. Biochimie 2015, 113, 143-155.
23. “Copper-Binding Tripeptide Motif Increases Potency of the Antimicrobial Peptide Anoplin via Reactive Oxygen Species Generation” Libardo, M. D. J.; Nagella, S.; Lugo, A.; Pierce, S.; Angeles-Boza, A. M. Biochem. Biophys. Rese. Comm. 2015, 456, 446-451.
22. “Bioinorganic Chemistry of Antimicrobial and Host-Defense Peptides” Libardo, M. D. J.; Angeles-Boza, A. M. Comments on Inorganic Chemistry 2014, 34, 42-58.
21. “Improved Bioactivity of Antimicrobial Peptides by Addition of Amino-Terminal Copper and Nickel (ATCUN) Binding Motifs” Libardo, M. D.; Cervantes, J. L.; Salazar, J. C.; Angeles-Boza, A. M.* ChemMedChem 2014, 9, 1892-1901.
Funding for this project: